Sunscreen Update: FDA Regulations and Your 2025 Skincare Routine

The sunscreen industry is undergoing a major shift as the FDA regulations prepares to implement new regulations by 2025. These updates aim to improve product safety, labeling transparency, and overall consumer trust.
Reformulations and stricter standards will require manufacturers to comply with updated testing and ingredient guidelines. This will affect which products remain on the shelves and how users choose their sun protection.
Understanding these changes now can help you make more confident and healthier skincare decisions going forward.
Understanding the FDA’s Role in Sunscreen Regulation
The FDA plays a central role in ensuring that sunscreens are safe, effective, and clearly labeled. As part of its public health mission, the agency regulates these products under strict pharmaceutical guidelines.
It reviews ingredients, oversees manufacturing practices, and verifies the claims made on sunscreen packaging. This ensures consumers receive reliable protection from UV damage when using approved products.
By strengthening oversight, the FDA regulations helps maintain high safety standards across all sun care options available in the U.S.
FDA’s Testing and Approval Process
Before a sunscreen reaches store shelves, it must undergo rigorous FDA evaluation to confirm safety and effectiveness. The process involves several detailed steps to ensure consumer protection.
-
Ingredient Review: Active ingredients are analyzed for safety, including risks of irritation, absorption, and long-term effects.
-
Efficacy Testing: Products must prove SPF and broad-spectrum claims through validated laboratory protocols.
-
Manufacturing Standards: Facilities are inspected for cleanliness, consistency, and regulatory compliance throughout production.
Each step helps confirm that sunscreen products offer dependable defense against harmful sun exposure.
The Significance of Broad-Spectrum Protection
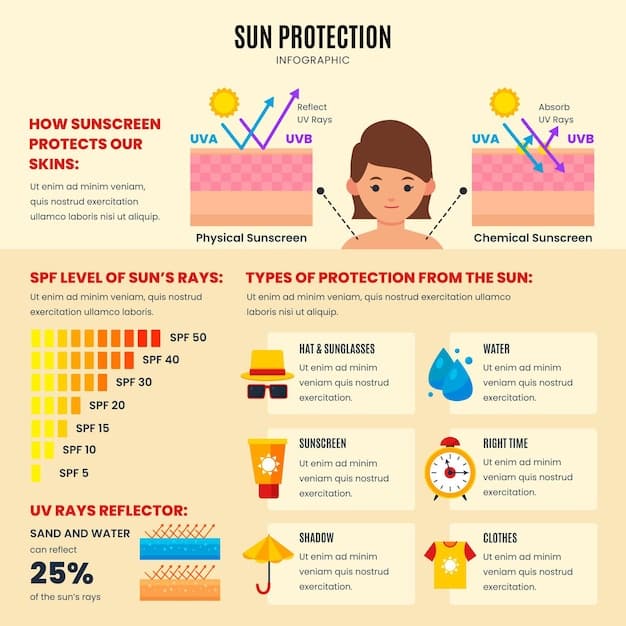
Broad-spectrum sunscreen plays a key role in protecting the skin from both UVA and UVB rays. These forms of radiation damage the skin differently, making dual protection essential for health.
UVA rays can accelerate signs of aging and increase cancer risk, while UVB rays cause visible sunburn and surface damage. Without both forms of protection, sunscreen remains incomplete.
FDA regulations now require sunscreens labeled “broad-spectrum” to meet specific performance standards that address both types of radiation.

Key Changes in the 2025 Regulations
The 2025 updates introduce a series of adjustments focused on transparency, ingredient safety, and product effectiveness. These changes aim to build consumer confidence and encourage smarter sunscreen choices.
Manufacturers will need to meet stricter testing standards, submit additional safety data, and update labels for clarity. This means some products may change, and others may be removed.
Understanding these updates helps consumers adapt their skincare routines to stay aligned with the latest sun protection science.
New Ingredient Safety Requirements
Ingredient safety is a key focus of the new FDA regulations and rules. Certain chemical filters are under review due to concerns about health and environmental impact.
-
Ingredient Re-evaluation: Ingredients like oxybenzone and octinoxate are being reassessed for hormone disruption and ecological effects.
-
New Safety Data: Companies must provide in-depth research on absorption, metabolism, and long-term skin impact.
-
Potential Restrictions: Some filters may face usage limits or warning labels depending on their updated safety profiles.
These efforts aim to ensure that only the safest and most effective filters remain in widespread use.
Updated Testing Protocols
The FDA regulations is also enhancing testing protocols to improve accuracy and consistency across sunscreen products. These updates will help ensure better protection and quality assurance.
-
Standardized SPF Testing: New guidelines will increase reliability in how SPF is measured and reported on packaging.
-
Broad-Spectrum Testing Enhancements: More precise methods will assess protection against UVA rays across a wider range of wavelengths.
This ensures consumers get what they expect from SPF ratings and broad-spectrum labels with every application.
How These Regulations Impact Your Skincare Routine
As regulations change, your approach to sun protection may need to adjust. Staying informed helps you make better product choices and develop healthier habits.
Many current sunscreens may be reformulated, discontinued, or relabeled in response to the new rules. Reading labels carefully will become even more important.
Let’s explore what you should consider when selecting sunscreen and applying it properly under the updated guidelines.
Choosing the Right Sunscreen
Selecting the right sunscreen starts with understanding the label and knowing which ingredients offer reliable protection. Mineral options are gaining popularity for their safety.
-
Read Labels Carefully: Choose broad-spectrum products with safe, FDA-compliant ingredients and adequate SPF.
-
Consider Mineral Sunscreens: Zinc oxide and titanium dioxide offer effective protection and low skin absorption.
These options are especially useful for people with sensitive skin or concerns about chemical UV filters in their routine.
Adjusting Your Application Habits
Even the best sunscreen requires proper application to be effective. Many users apply too little or forget to reapply throughout the day.
-
Apply Generously: Use about one ounce to fully cover your body, and ensure even coverage across all exposed areas.
-
Reapply Frequently: Every two hours or after swimming and sweating to maintain consistent protection levels.
By improving your technique, you ensure that the sunscreen performs as intended and offers the advertised level of protection.
Navigating the Transition: What Consumers Need to Know
Transitioning to the new regulatory landscape will take time, but being proactive makes the shift easier. Consumers who stay informed can avoid confusion and buy with confidence.
Some familiar products may disappear or look different due to updated formulations. Check for FDA-compliant ingredients and clear labeling as new products enter the market.
Let’s look at practical steps you can take to adapt your routine without compromising sun safety.
Staying Informed
Accessing reliable information is key to keeping up with the latest changes. Rely on official sources and professional advice to guide your decisions.
-
Check FDA Resources: Visit the official FDA website for updates on sunscreen regulations, product recalls, and ingredient safety.
-
Consult Healthcare Professionals: Dermatologists can offer personalized recommendations based on your skin type and lifestyle.
These steps help you stay ahead of industry changes and make informed skincare choices as regulations evolve.
Addressing Common Concerns
Some consumers worry that reformulated products may be less effective or harder to find. Being aware of these concerns helps you prepare and adapt accordingly.
-
Ingredient Availability: Some filters may be limited or phased out, requiring consumers to switch to safer alternatives.
-
Product Performance: Look for sunscreens that meet updated SPF and broad-spectrum testing requirements to ensure reliability.
Trustworthy brands will adjust their formulas to remain compliant while maintaining high standards of performance and protection.
The Future of Sunscreen Technology and Innovation
The 2025 changes are driving innovation as companies develop safer, more advanced sunscreen technologies. With new filters and delivery methods in progress, the industry is moving toward smarter and more reliable protection.
These innovations focus on improving both skin compatibility and overall user experience. The goal is to create sunscreens that are more comfortable to wear, easier to apply, and safer for long-term use.
This new phase in sun care marks a shift toward products that combine high performance with sustainability, meeting modern consumer needs.
Emerging Ingredients and Formulations
Brands are moving toward ingredients that balance safety, stability, and environmental impact. Inorganic and next-gen organic filters lead the charge in safer formulations.
-
Encapsulation Technology: Filters enclosed in microcapsules reduce irritation, improve stability, and prevent deep skin absorption.
-
Water-Resistant Formulas: Advanced polymers increase longevity on skin, especially during water or sweat exposure.
These innovations offer long-lasting protection while enhancing comfort and reducing negative effects on skin or marine life.
The new FDA regulations regarding sunscreen update: new FDA regulations and how they impact your daily skincare routine in 2025 represent a significant step towards ensuring safer and more effective sun protection for consumers.
By staying informed about these changes, adjusting your skincare routine accordingly, and embracing the latest innovations in sunscreen technology, you can continue to protect your skin from the harmful effects of UV radiation.
| Key Point | Brief Description |
|---|---|
| ☀️ New FDA Regulations | Updates to sunscreen standards focusing on safety and efficacy by 2025. |
| 🧪 Ingredient Safety | Re-evaluation of chemical UV filters like oxybenzone for potential health impacts. |
| 🔬 Testing Protocols | Enhanced standards for SPF and broad-spectrum protection testing. |
| 🧴 Choosing Sunscreen | Focus on broad-spectrum, mineral-based options, and careful label reading. |
FAQ: Sunscreen and the New FDA Regulations
▼
The primary goals are to ensure that sunscreens are safe, effective, and accurately labeled. This includes re-evaluating ingredients and improving testing standards for broad-spectrum protection.
▼
Several chemical UV filters, like oxybenzone and octinoxate, are being re-evaluated due to concerns about potential hormone disruption and environmental impact. New safety data is required.
▼
Look for sunscreens labeled “broad-spectrum” with an SPF of 30 or higher. Apply generously and reapply every two hours, especially after swimming or sweating.
▼
Mineral sunscreens, using zinc oxide and titanium dioxide, are generally considered safe. They are a good alternative for those concerned about chemical UV filters.
▼
The FDA’s website is the best source for accurate information on sunscreen regulations, ingredient safety, and proper usage. Consult healthcare professionals for personalized advice.
Understanding the new FDA regulations is key to ensuring that the sunscreens you use are safe and effective.
These updates aim to increase transparency in labeling and enhance consumer confidence in sun protection products.
As the 2025 implementation date nears, staying informed and using the right products with proper application will help you protect your skin effectively. Early awareness leads to smarter choices and better long-term care.





